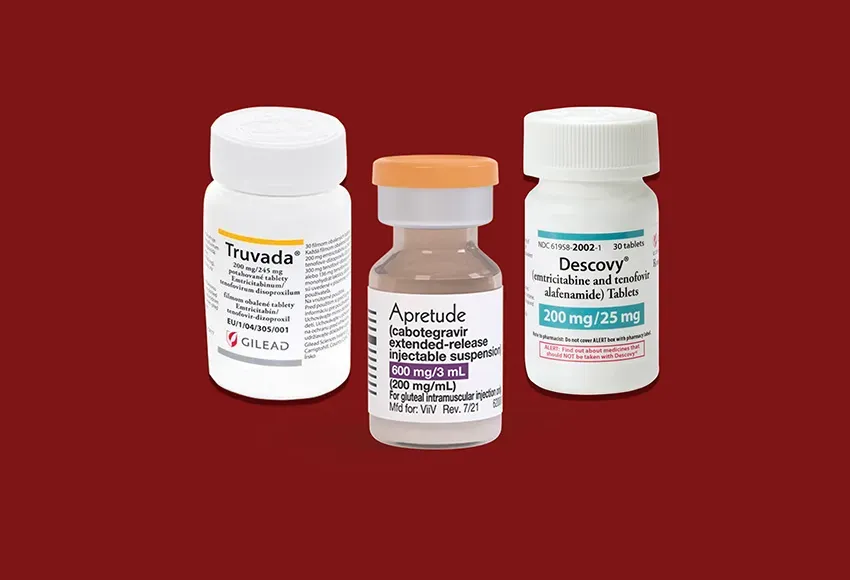
A worldwide study completed by the US Preventive Services Task Force has led to three PrEP medications being approved for people at risk for HIV/AIDS.
PrEP, or pre-exposure prophylaxis, is a type of medication that reduces the chances of contracting HIV from sex or injection drug use.
According to Infectious Disease Special Edition, the medications are "the daily oral agent tenofovir disoproxil fumarate with emtricitabine (Truvada, Gilead) ... and the daily oral drug tenofovir alafenamide with emtricitabine (Descovy, Gilead) and the long-acting injectable cabotegravir (Apretude, ViiV Healthcare)."
In the conclusion of the 2023 study titled "Preexposure Prophylaxis for the Prevention of HIV: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force," the authors state that "in adults at increased HIV acquisition risk, oral PrEP was associated with decreased risk of acquiring HIV infection compared with placebo or no PrEP. Oral TAF/FTC was noninferior to oral TDF/FTC, and injectable cabotegravir reduced the risk of HIV infection compared with oral TDF/FTC in the populations studied."
According to the USPSTF, 1.2 million people in the US are eligible to receive PrEP medication to prevent the spread of HIV/AIDS, but only 30% of those people are taking it. This is due in part to existing disparities and issues with obtaining access to the drug, which is especially true in communities of color. According to the Centers for Disease Control and Prevention, only 11% of eligible Black people and 21% of eligible Latinx people take PrEP, compared to 78% of eligible white people.
One of the hopes of the USPSTF is to increase accessibility to PrEP, because it has been proven to be successful. But unfortunately, due to a history of stigma related to HIV/AIDS and a general mistrust for the healthcare system, many people choose not to take the drug, which is also not always covered completely by insurance. Another issue is the fact that many people who are eligible to take the drug are unable to obtain refills, due to preexisting issues like substance abuse or being unhoused.
According to the USPSTF, "an estimated 1.2 million persons in the US currently have HIV and more than 760,000 persons have died of complications related to HIV since 1981."
Access to PrEP in Washington state
Washington state has a PrEP drug assistance program (DAP) that provides financial assistance for HIV-negative people who have risk factors that expose them to HIV.
According to the Washington State Department of Health, in order to be eligible for PrEP DAP you must:
1. Be HIV-negative
2. Live in Washington state
3. Meet one of the following risk factors:
a. Is male or Transgender and has sex with men and has one or more of the following risks:
• diagnosis of rectal or urethral gonorrhea, rectal chlamydia, or early syphilis in the prior 12 months
• methamphetamine or popper use in the prior 12 months
• history of providing sex for money, drugs, food, shelter, or transportation in the prior 12 months
• unprotected anal sex outside of a long-term, mutually monogamous relationship
b. Is in an ongoing sexual relationship with an HIV-infected person who:
• is not on antiretroviral therapy (ART)
• is on ART but is not virologically suppressed
• is within six months of initiating ART
• is on ART and is virologically suppressed
c. Is in an ongoing sexual relationship in which the female partner is trying to get pregnant
d. Is a woman who provides sex for money, drugs, food, shelter, or transportation
e. Injects drugs that are not prescribed by a medical provider
For more information regarding eligibility for Washington's PrEP DAP, go to https://doh.wa.gov/you-and-your-family/illness-and-disease-z/hiv/prevention/pre-exposure-prophylaxis-drug-assistance-program-prep-dap